BONDING AT O°C
- GROUP 2 CHEM

- Oct 13, 2018
- 3 min read
HAVE YOU EVER TRIED ICE SKATING ON A FROZEN LAKE?
If yes, then you could have asked yourself at least once, how does the surface of a lake turn into solid while the water beneath remains liquid? Or, how do the marine life below are still alive? Ice is an interesting material and HYDROGEN BONDING is the reason for its unique property.
Hydrogen Bonding is the strongest among intermolecular bonds. It is the partially electrostatic attraction between Hydrogen (H) to a more electronegative atom namely Nitrogen (N), Oxygen (O) and Fluorine (F). Want to learn more about it? Let’s go!
"In terms of the Lewis theory, a free pair of electrons on one water molecule might be able to exert sufficient force on a hydrogen held by a pair of electrons on another water molecule to bind the two molecules together. Structurally this may be represented as " ~ Worth Rodebush (1887 – 1959)
Ice is formed from water after a decrease in temperature at a particular pressure through the process of freezing. Hydrogen bonding doesn’t necessarily have a role in its formation so the conversion of water into ice is still very much possible (Jha,2017). However, since it affects it structure, density and freezing temperature, without hydrogen bonding, ice would have a very different physical structure.

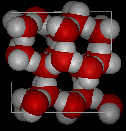
During its liquid phase, the molecules are clustered up together, having lesser volume thus higher density. When the temperature of water is lowered and it freezes, its molecules form a crystalline structure maintained by hydrogen bonding since there is a lack of energy to break the bond. As a result, the existing hydrogen bond, the structure of the ice is a rigid lattice structure. Atoms are arranged at the corner of a puckered hexagonal rings (Britannica, 2018). This is why snowflakes are not in a shape of a square, because it is mirroring a reflections of the molecular structure of ice.

As you can see in the figure in the left, the structure of ice has spaces, somewhat like an open-cage structure with lots of empty space inside. Because of this, the density of ice is less than liquid water. This is a unique characteristic of ice since it is a phenomenon not seen in the solidification of other liquids. Generally, the freezing of other liquids showcased the decrease of kinetic energy between its molecules allowing them to be in more contact with one another unlike in their liquid form (Petrucci, 2007). This causes a decrease in volume which will result in an increase in density. However, in ice, the density lessens by about 10% as liquid water solidifies. (Kotz et.al, 2018).
Since materials with lower density will float while ones with higher density will sink, the liquid water sinks down and the ice climbs up in the surface of a liquid water. Such phenomenon is seen in icebergs in the middle of the ocean on cold regions or ice cubes in a glass of water (Kotz et.al, 2018). Moreover, during winter, the ice that is formed in the surface of lakes and ponds creates an insulating barrier protecting the marine life living in the liquid water below. Without this, the marine life living beneath the water will freeze to death and will not survive (Cracolice et. al., 2006).
This is the beauty of hydrogen bonds in ice. As said above, without hydrogen bonding, the solid phase of water will have a very different structure. Imagine if this structure is formed from other bonding, then there is a possibility that it would not have the effect of having empty spaces within its molecules, just like it did with hydrogen bonding. This will then increase the density of ice. This will result in the sinking of ice, thus killing marine life every winter and changing the vast oceans and seas as we know it. Life on Earth would be complicated, or worse, impossible! So let’s continue to appreciate the beauty of hydrogen bonding that exist with mother nature and its significant contributions that allow us to live in this world.

















Comments